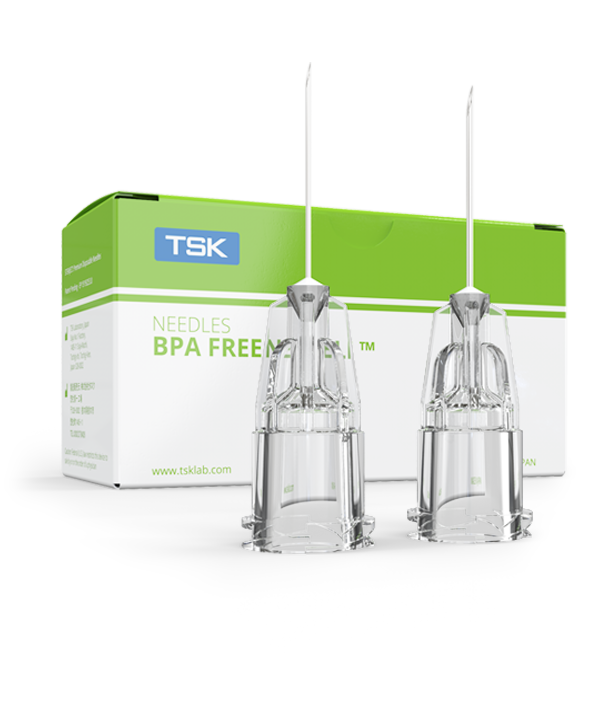
TSK Laboratory introduces the first BPA FREE NEEDLE.
The common use of epoxy in the manufacturing of hypodermic needles contains BPA or BADGE. The potential adverse effects have been reported as a result from the release of these uncured epoxy resin particles, and there continues to be uncertainties concerning the risks for patients and medical personnel.The health risks associated with excessive adhesive particles and plastic particles entering the blood stream is judged to be very low during normal use, but it can reach unacceptable levels in extreme situations (i.e. pediatric intensive care units).
Another area that is being investigated is whether the use of titanium dioxide TiO2 is actually needed as a coloring agent. It is unknown what the consequences are for the safety of patients as information on this is lacking in literature and little is known about possible exposure via hypodermic needles.Needle manufacturers, notified bodies and competent authorities are responsible for continuously improving the safety and risk for public health, but according to the ISO 7864 standard for hypodermic injection needles there are no limitations to the amount of glue in hypodermic needles nor has there been any regulation around BPA.
TSK Laboratory, one of the leading hypodermic needle manufacturers, has been proactive with new production techniques to design the BPA FREE NEEDLE ™. This BPA FREE NEEDLE ™ is produced using only safe components in production, free from Bisphenol A, BADGE and titanium dioxide.


Advanced hub geometry.
Best possible flow rate.
More controlled injection.
Dermal fillers have a high viscosity and contain small particles that make injections more difficult. The BPA FREE hub has a wider internal geometry for improved flow rates. The BPA FREE hub also has an overall shorter length than the PRC hub.





 | TSK Laboratory International ©
| TSK Laboratory International ©
